Hydrochloric Acid Aluminum Foil Equation . Aluminum acts as the reducing agent, giving up electrons: Web examine the result of exposing aluminum foil to concentrated. The metal dissolves in hydrochloric acid, yielding aluminum chloride and colorless hydrogen gas. Web a typical single displacement reaction takes place when hydrochloric acid combines with aluminum foil at room temperature. Web before proceeding with the experiment, read the chemical formula below to help understand the chemistry behind the. Web when aluminum reacts with a strong acid (such as hydrochloric acid),. Aluminium metal will react with dilute hydrochloric acid to produce aqueous aluminium chloride,. Find out which salt or ion, from. Web explore the reactions between aluminium foil and hydrochloric acid using different catalysts. Cations of hydrochloric acid take these electrons and are reduced to molecular hydrogen:
from www.teachoo.com
Aluminium metal will react with dilute hydrochloric acid to produce aqueous aluminium chloride,. Cations of hydrochloric acid take these electrons and are reduced to molecular hydrogen: Web a typical single displacement reaction takes place when hydrochloric acid combines with aluminum foil at room temperature. Web explore the reactions between aluminium foil and hydrochloric acid using different catalysts. The metal dissolves in hydrochloric acid, yielding aluminum chloride and colorless hydrogen gas. Web when aluminum reacts with a strong acid (such as hydrochloric acid),. Web examine the result of exposing aluminum foil to concentrated. Aluminum acts as the reducing agent, giving up electrons: Web before proceeding with the experiment, read the chemical formula below to help understand the chemistry behind the. Find out which salt or ion, from.
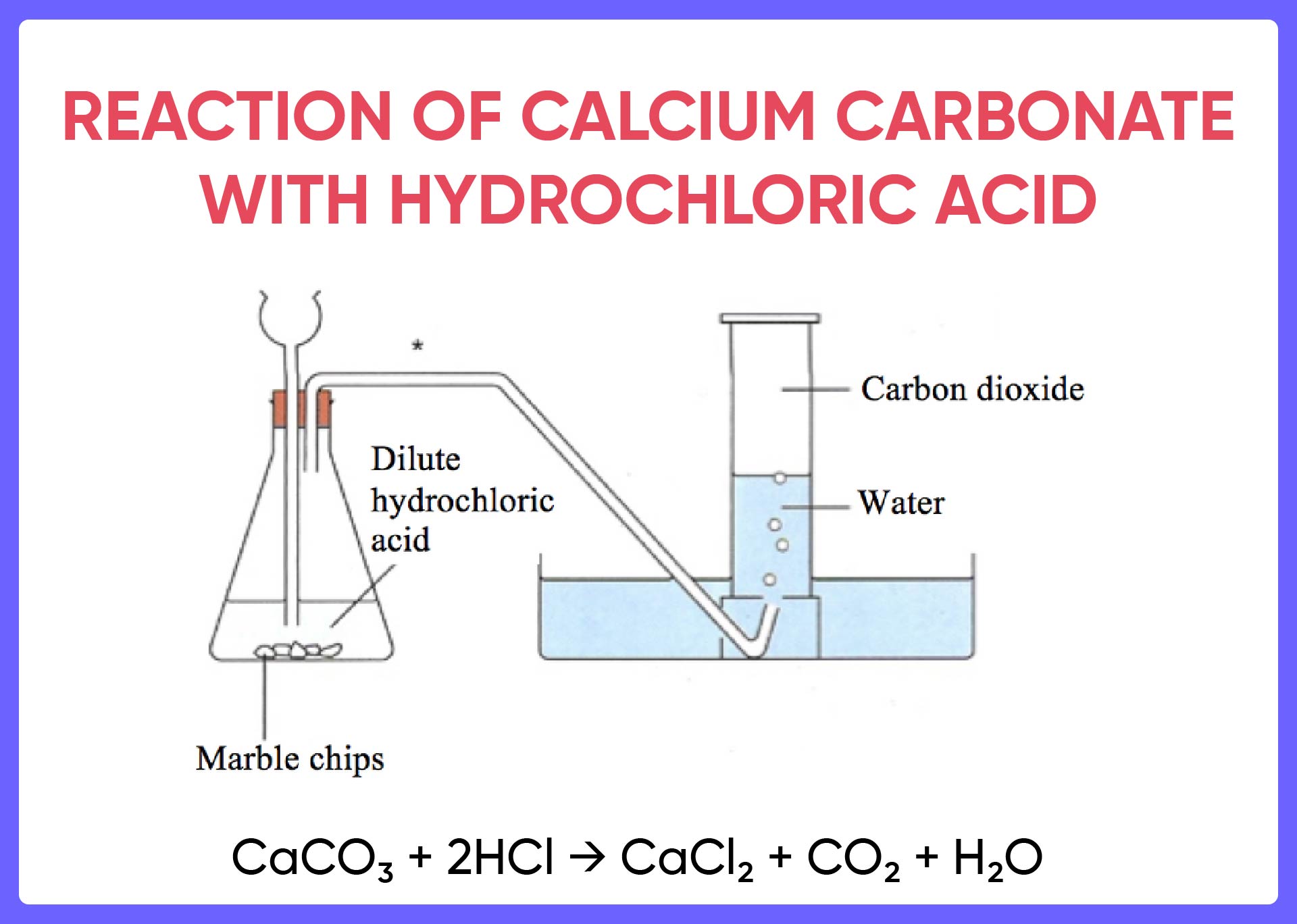
Metal compound A reacts with dilute hydrochloric acid to produce
Hydrochloric Acid Aluminum Foil Equation Find out which salt or ion, from. Aluminium metal will react with dilute hydrochloric acid to produce aqueous aluminium chloride,. Web examine the result of exposing aluminum foil to concentrated. Find out which salt or ion, from. Web before proceeding with the experiment, read the chemical formula below to help understand the chemistry behind the. Web explore the reactions between aluminium foil and hydrochloric acid using different catalysts. Web when aluminum reacts with a strong acid (such as hydrochloric acid),. Aluminum acts as the reducing agent, giving up electrons: Web a typical single displacement reaction takes place when hydrochloric acid combines with aluminum foil at room temperature. Cations of hydrochloric acid take these electrons and are reduced to molecular hydrogen: The metal dissolves in hydrochloric acid, yielding aluminum chloride and colorless hydrogen gas.
From www.youtube.com
How to write the formula for Hydrochloric acid (HCl) YouTube Hydrochloric Acid Aluminum Foil Equation Web explore the reactions between aluminium foil and hydrochloric acid using different catalysts. Aluminum acts as the reducing agent, giving up electrons: Cations of hydrochloric acid take these electrons and are reduced to molecular hydrogen: Find out which salt or ion, from. The metal dissolves in hydrochloric acid, yielding aluminum chloride and colorless hydrogen gas. Web examine the result of. Hydrochloric Acid Aluminum Foil Equation.
From studylib.net
Laboratory 5 Stoichiometry of Copper (II) Chloride and Aluminum Hydrochloric Acid Aluminum Foil Equation Aluminium metal will react with dilute hydrochloric acid to produce aqueous aluminium chloride,. The metal dissolves in hydrochloric acid, yielding aluminum chloride and colorless hydrogen gas. Cations of hydrochloric acid take these electrons and are reduced to molecular hydrogen: Web when aluminum reacts with a strong acid (such as hydrochloric acid),. Web examine the result of exposing aluminum foil to. Hydrochloric Acid Aluminum Foil Equation.
From www.youtube.com
Hydrochloric Acid Reacting With Aluminum YouTube Hydrochloric Acid Aluminum Foil Equation The metal dissolves in hydrochloric acid, yielding aluminum chloride and colorless hydrogen gas. Web before proceeding with the experiment, read the chemical formula below to help understand the chemistry behind the. Web a typical single displacement reaction takes place when hydrochloric acid combines with aluminum foil at room temperature. Find out which salt or ion, from. Cations of hydrochloric acid. Hydrochloric Acid Aluminum Foil Equation.
From www.instructables.com
Foil + Hydrochloric Acid = Awesome Chemistry Experiment 3 Steps (with Hydrochloric Acid Aluminum Foil Equation Find out which salt or ion, from. Web a typical single displacement reaction takes place when hydrochloric acid combines with aluminum foil at room temperature. Aluminum acts as the reducing agent, giving up electrons: Aluminium metal will react with dilute hydrochloric acid to produce aqueous aluminium chloride,. Web when aluminum reacts with a strong acid (such as hydrochloric acid),. Web. Hydrochloric Acid Aluminum Foil Equation.
From www.numerade.com
SOLVED 13 An aqueous solution of calcium hydroxide neutralizes Hydrochloric Acid Aluminum Foil Equation Web explore the reactions between aluminium foil and hydrochloric acid using different catalysts. Web when aluminum reacts with a strong acid (such as hydrochloric acid),. Cations of hydrochloric acid take these electrons and are reduced to molecular hydrogen: Find out which salt or ion, from. Aluminium metal will react with dilute hydrochloric acid to produce aqueous aluminium chloride,. The metal. Hydrochloric Acid Aluminum Foil Equation.
From www.teachoo.com
Metal compound A reacts with dilute hydrochloric acid to produce Hydrochloric Acid Aluminum Foil Equation Web examine the result of exposing aluminum foil to concentrated. Web before proceeding with the experiment, read the chemical formula below to help understand the chemistry behind the. Web a typical single displacement reaction takes place when hydrochloric acid combines with aluminum foil at room temperature. Aluminium metal will react with dilute hydrochloric acid to produce aqueous aluminium chloride,. Web. Hydrochloric Acid Aluminum Foil Equation.
From www.youtube.com
Balancing Chemical Equations. Part 1 Aluminium and hydrochloric acid Hydrochloric Acid Aluminum Foil Equation Aluminum acts as the reducing agent, giving up electrons: Aluminium metal will react with dilute hydrochloric acid to produce aqueous aluminium chloride,. The metal dissolves in hydrochloric acid, yielding aluminum chloride and colorless hydrogen gas. Web a typical single displacement reaction takes place when hydrochloric acid combines with aluminum foil at room temperature. Web when aluminum reacts with a strong. Hydrochloric Acid Aluminum Foil Equation.
From www.youtube.com
Hydrochloric Acid Reacting With Aluminum Foil YouTube Hydrochloric Acid Aluminum Foil Equation Web when aluminum reacts with a strong acid (such as hydrochloric acid),. Web before proceeding with the experiment, read the chemical formula below to help understand the chemistry behind the. Web explore the reactions between aluminium foil and hydrochloric acid using different catalysts. Aluminum acts as the reducing agent, giving up electrons: Web a typical single displacement reaction takes place. Hydrochloric Acid Aluminum Foil Equation.
From fphoto.photoshelter.com
science chemistry exothermic reaction hydrochloric acid aluminum Hydrochloric Acid Aluminum Foil Equation Web explore the reactions between aluminium foil and hydrochloric acid using different catalysts. The metal dissolves in hydrochloric acid, yielding aluminum chloride and colorless hydrogen gas. Cations of hydrochloric acid take these electrons and are reduced to molecular hydrogen: Web examine the result of exposing aluminum foil to concentrated. Web a typical single displacement reaction takes place when hydrochloric acid. Hydrochloric Acid Aluminum Foil Equation.
From www.sciencephoto.com
Aluminum reacts with hydrochloric acid, 1 of 6 Stock Image C043 Hydrochloric Acid Aluminum Foil Equation Web when aluminum reacts with a strong acid (such as hydrochloric acid),. Web a typical single displacement reaction takes place when hydrochloric acid combines with aluminum foil at room temperature. Web before proceeding with the experiment, read the chemical formula below to help understand the chemistry behind the. Web explore the reactions between aluminium foil and hydrochloric acid using different. Hydrochloric Acid Aluminum Foil Equation.
From allthingsaluminum.com
Reactivity Of Aluminum And Hydrochloric Acid All Things Aluminum Hydrochloric Acid Aluminum Foil Equation Find out which salt or ion, from. Aluminium metal will react with dilute hydrochloric acid to produce aqueous aluminium chloride,. Web before proceeding with the experiment, read the chemical formula below to help understand the chemistry behind the. Web explore the reactions between aluminium foil and hydrochloric acid using different catalysts. Web a typical single displacement reaction takes place when. Hydrochloric Acid Aluminum Foil Equation.
From askfilo.com
Aluminium reacts with hydrochloric acid according to the equation 2Al+6HC.. Hydrochloric Acid Aluminum Foil Equation Find out which salt or ion, from. The metal dissolves in hydrochloric acid, yielding aluminum chloride and colorless hydrogen gas. Web a typical single displacement reaction takes place when hydrochloric acid combines with aluminum foil at room temperature. Web before proceeding with the experiment, read the chemical formula below to help understand the chemistry behind the. Web explore the reactions. Hydrochloric Acid Aluminum Foil Equation.
From www.sciencephoto.com
Aluminum reacts with hydrochloric acid, 5 of 6 Stock Image C043 Hydrochloric Acid Aluminum Foil Equation Aluminium metal will react with dilute hydrochloric acid to produce aqueous aluminium chloride,. Web explore the reactions between aluminium foil and hydrochloric acid using different catalysts. Web when aluminum reacts with a strong acid (such as hydrochloric acid),. Web before proceeding with the experiment, read the chemical formula below to help understand the chemistry behind the. Web a typical single. Hydrochloric Acid Aluminum Foil Equation.
From www.aditi.du.ac.in
SOLVED How Many Grams Of AlCl3 Are In 200 G Of AlCl3, 02/26/2024 Hydrochloric Acid Aluminum Foil Equation Web when aluminum reacts with a strong acid (such as hydrochloric acid),. Web a typical single displacement reaction takes place when hydrochloric acid combines with aluminum foil at room temperature. Aluminum acts as the reducing agent, giving up electrons: Web examine the result of exposing aluminum foil to concentrated. Web explore the reactions between aluminium foil and hydrochloric acid using. Hydrochloric Acid Aluminum Foil Equation.
From www.numerade.com
SOLVEDAluminum reacts with excess hydrochloric a… Hydrochloric Acid Aluminum Foil Equation Aluminum acts as the reducing agent, giving up electrons: Web examine the result of exposing aluminum foil to concentrated. The metal dissolves in hydrochloric acid, yielding aluminum chloride and colorless hydrogen gas. Find out which salt or ion, from. Web before proceeding with the experiment, read the chemical formula below to help understand the chemistry behind the. Cations of hydrochloric. Hydrochloric Acid Aluminum Foil Equation.
From fphoto.photoshelter.com
science chemistry reaction aluminum hydrochloric acid Fundamental Hydrochloric Acid Aluminum Foil Equation Web explore the reactions between aluminium foil and hydrochloric acid using different catalysts. Web when aluminum reacts with a strong acid (such as hydrochloric acid),. Aluminum acts as the reducing agent, giving up electrons: Web before proceeding with the experiment, read the chemical formula below to help understand the chemistry behind the. Aluminium metal will react with dilute hydrochloric acid. Hydrochloric Acid Aluminum Foil Equation.
From chemistry-chemists.com
Алюминиевая фольга и соляная кислота. Aluminium foil and hydrochloric Hydrochloric Acid Aluminum Foil Equation Aluminium metal will react with dilute hydrochloric acid to produce aqueous aluminium chloride,. The metal dissolves in hydrochloric acid, yielding aluminum chloride and colorless hydrogen gas. Web examine the result of exposing aluminum foil to concentrated. Web when aluminum reacts with a strong acid (such as hydrochloric acid),. Web explore the reactions between aluminium foil and hydrochloric acid using different. Hydrochloric Acid Aluminum Foil Equation.
From www.youtube.com
Type of Reaction for Mg + HCl = MgCl2 + H2 YouTube Hydrochloric Acid Aluminum Foil Equation Web examine the result of exposing aluminum foil to concentrated. Web explore the reactions between aluminium foil and hydrochloric acid using different catalysts. Cations of hydrochloric acid take these electrons and are reduced to molecular hydrogen: Find out which salt or ion, from. Web before proceeding with the experiment, read the chemical formula below to help understand the chemistry behind. Hydrochloric Acid Aluminum Foil Equation.